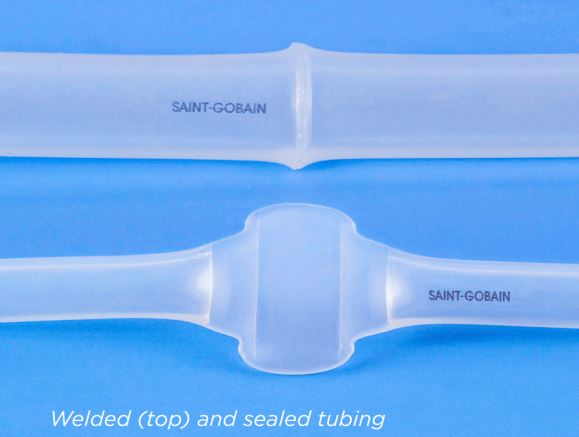
C-Flex – Ống hợp nhất khớp nối và seal
Features / Benefts
• Sealable and weldable either pre- or post-sterilization
• Sterilizable by gamma irradiation and autoclave
• Validation package available for all C-Flex® commercial formulations
• Moldable, bondable and formable for single-use assemblies and overmolds
• Non-pyrogenic, non-cytotoxic, non-hemolytic
• Remains flexible from -45ºC to 135ºC (-50ºF to 275ºF)
• Signifcantly less permeable than silicone
• Smooth inner bore for superior fluid flow
• All formulas are Animal-Derived Component Free
Single-use Applications
• Aseptic sealing disconnections
• Aseptic welding connections
• Ideal for use in single-use assemblies
• Buffer and media preparation
• Cell culture operations
• Purification operations
• Diagnostic products
• Biopharma manufacturing
• Single-use fluid transfer sets
• Tubing and bags manifolds
• Laboratory R&D
• High-purity water systems